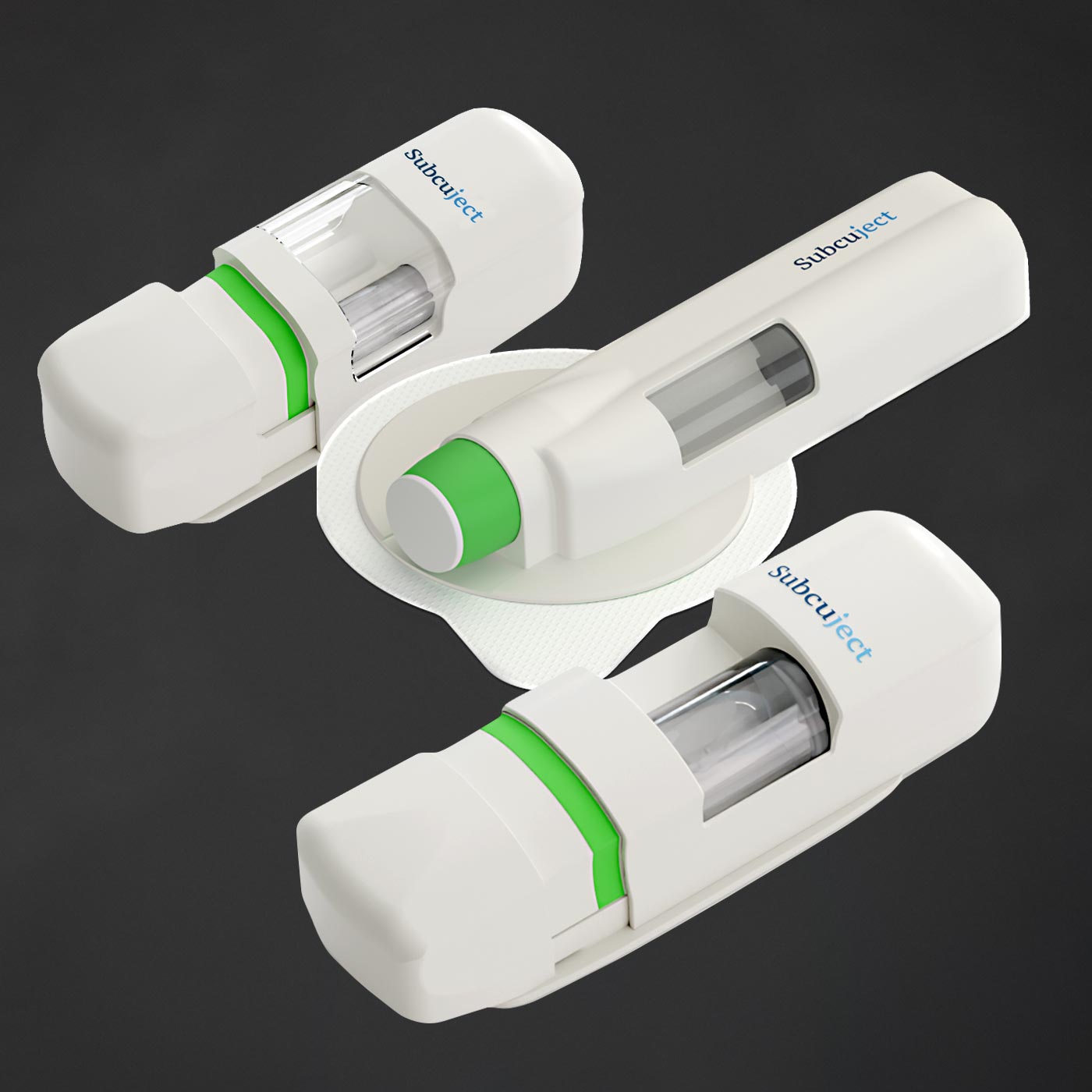
<1 ml
<3 ml
<10 ml
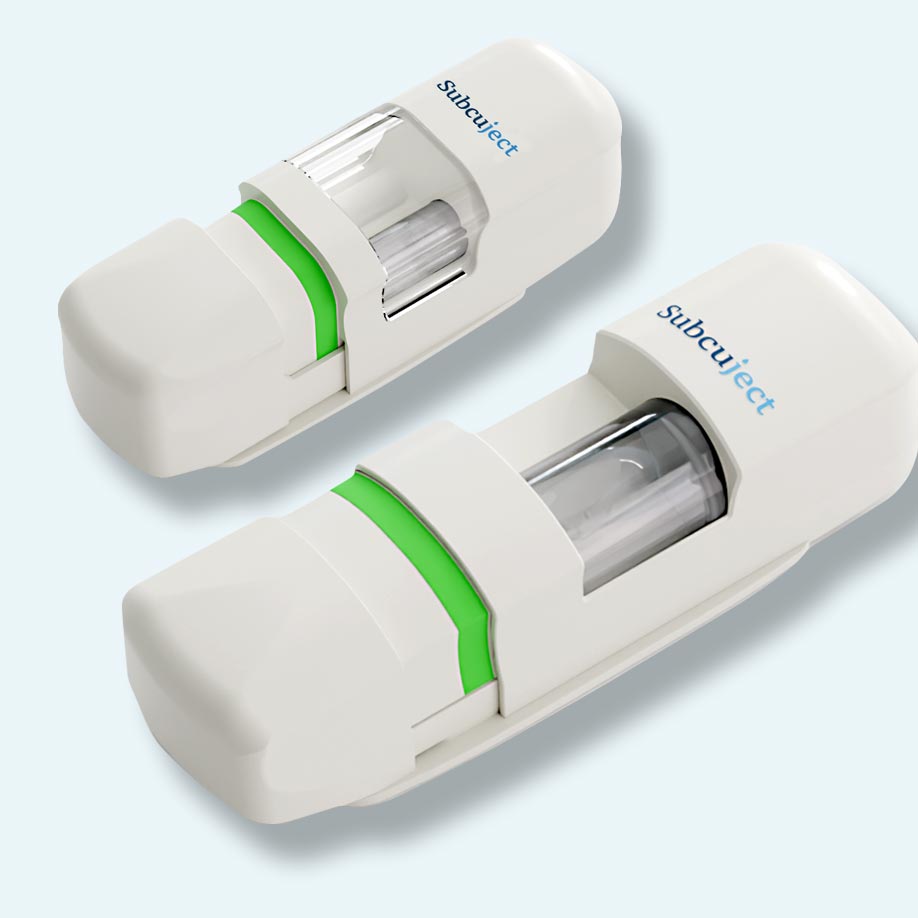
Benefits of Hands-Free Autoinjector
Injection volumes from < 1 ml to > 10 ml
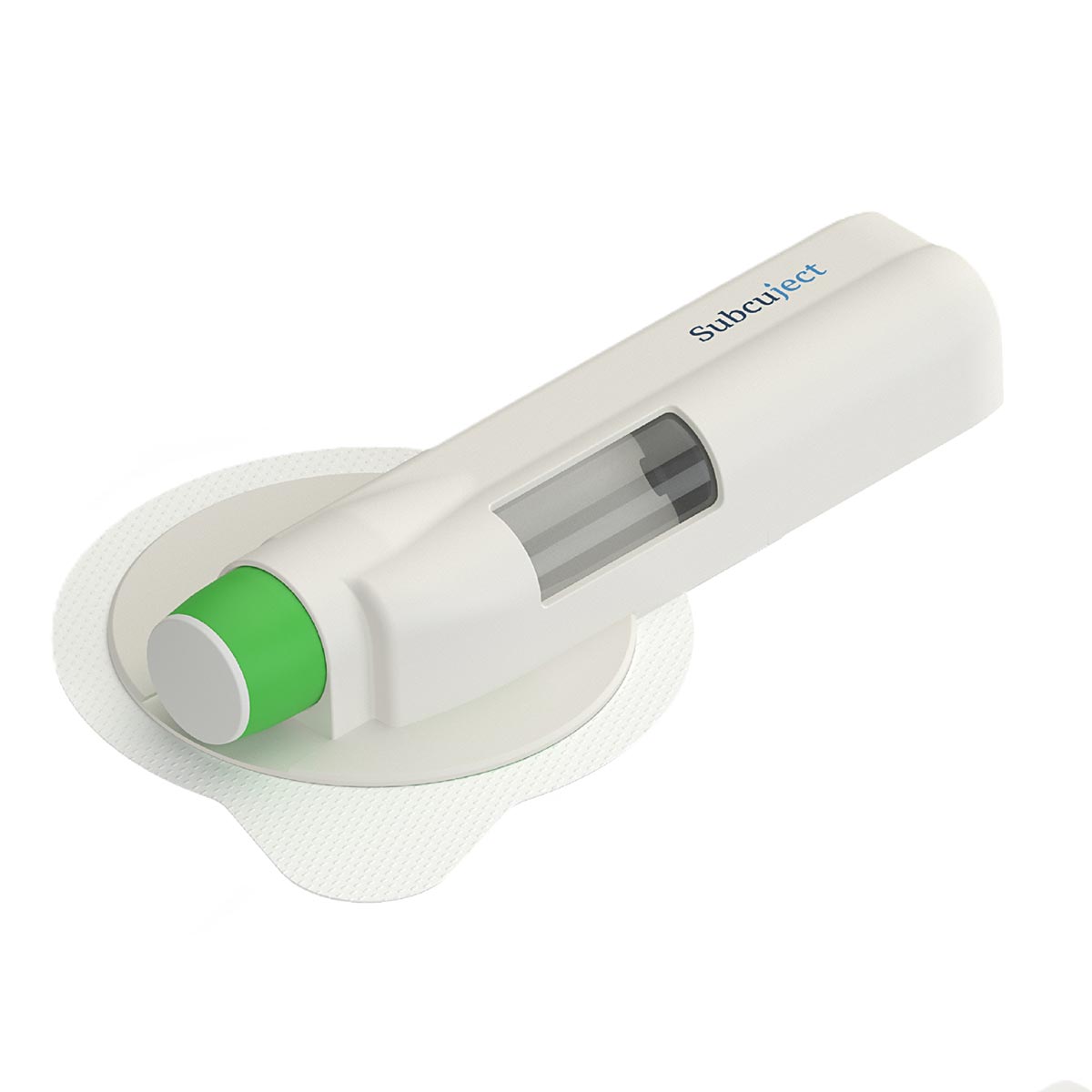
Use related
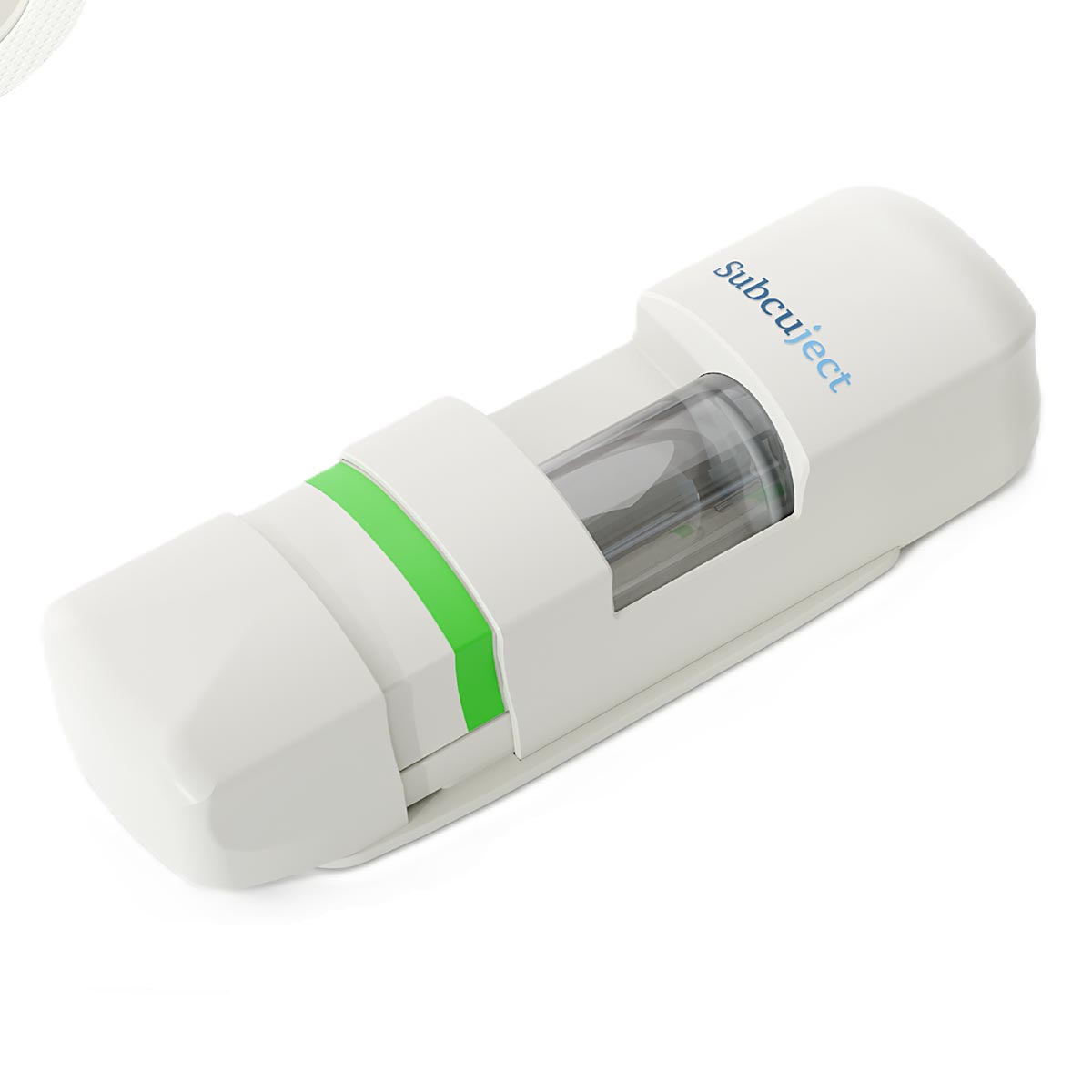
Technically
Why Hands-Free Autoinjectors
* Proprietary data from user preference studies demonstrate preference for Hand-Free Autoinjector among Rheumatoid Arthritis (RA) patients – even for low volume injection of adalimumab
Sequence of use/functionality
Intended as pre-filled, pre-loaded and ready to use
Pre injection
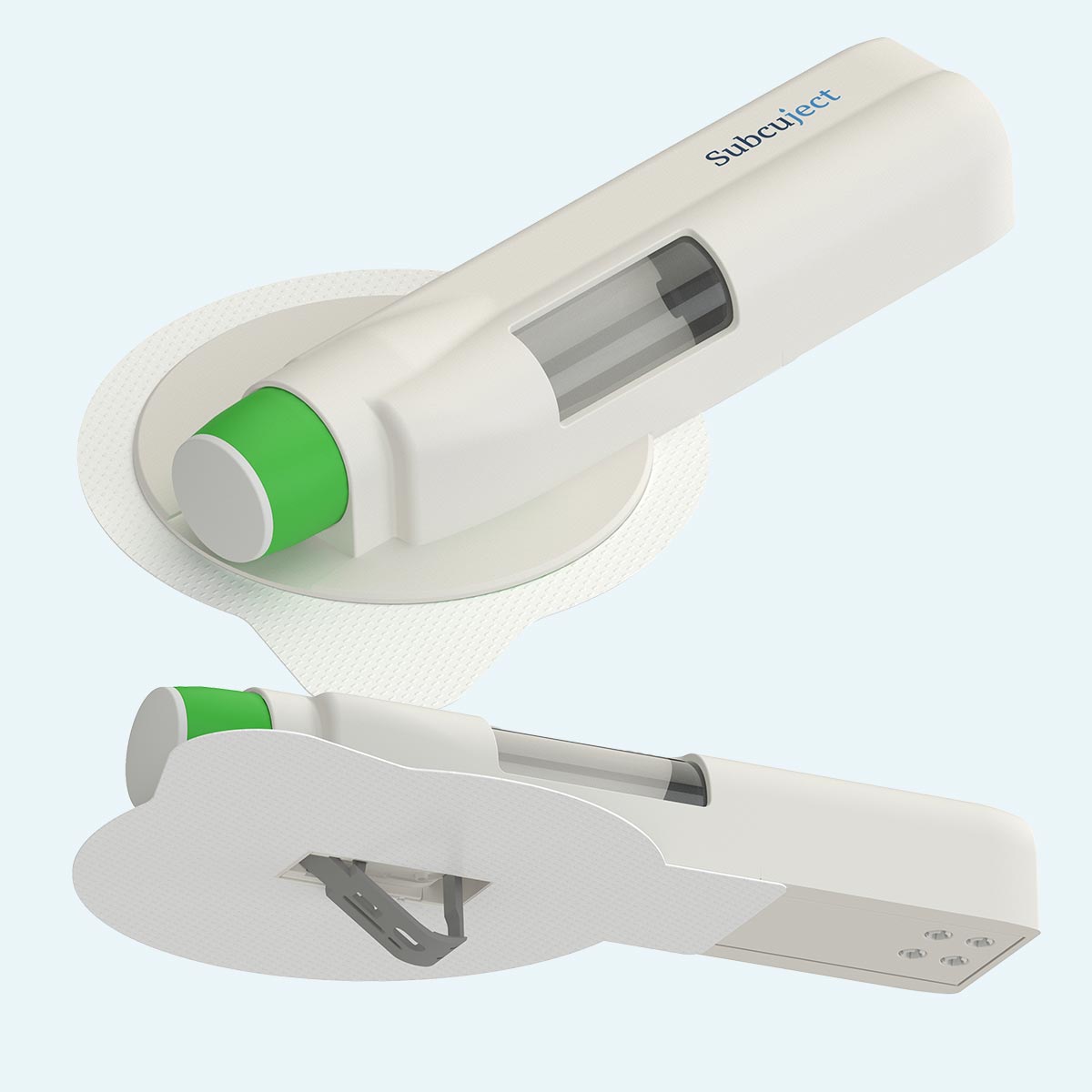
During injection
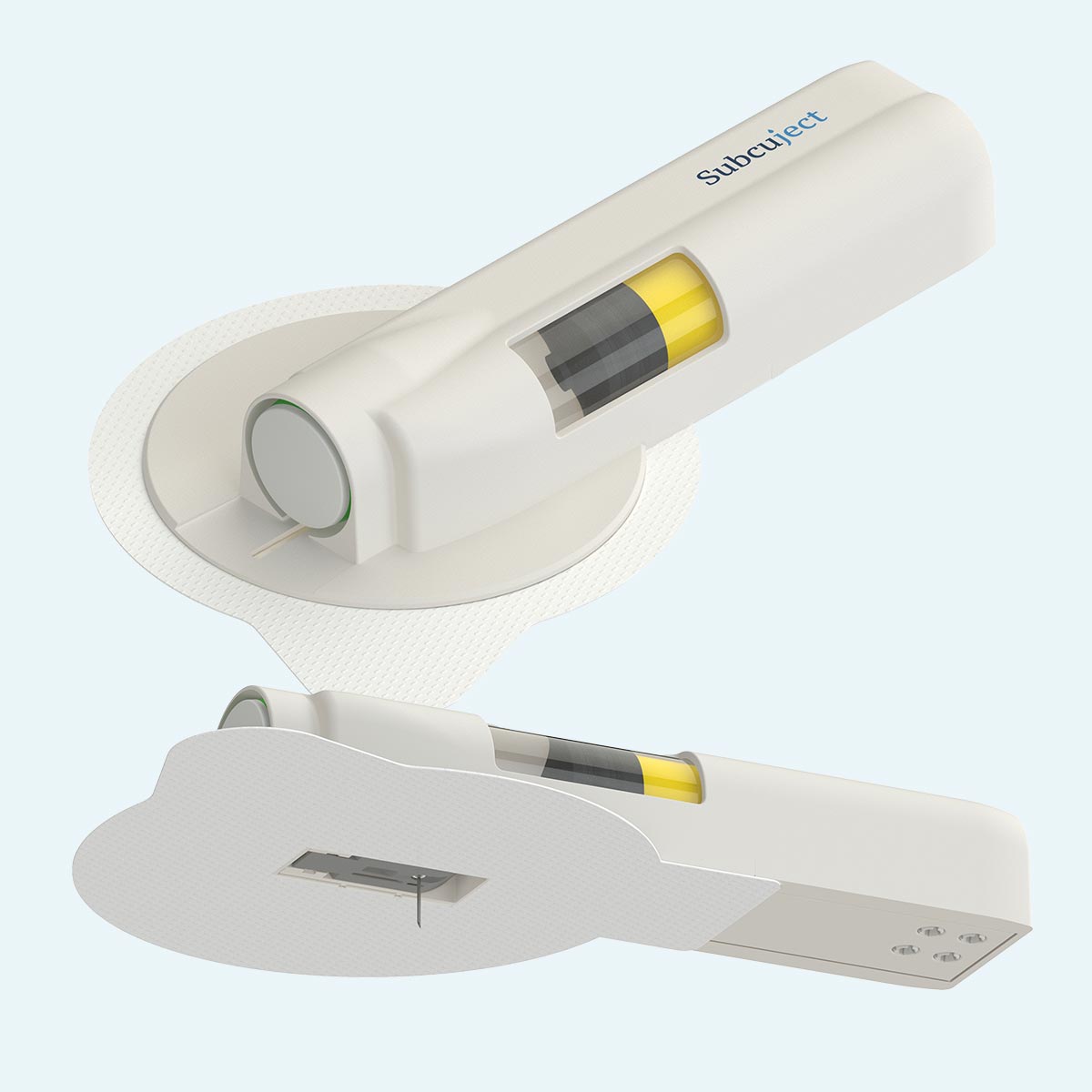
Post injection

Development stage
The concept is ready for regulatory development, feasibility evaluation and user studies

Collaboration
TJCC-Subcuject is seeking to out-license/divest IP to pharmaceutical- or device companies pursuing development of patient friendly combination solutions
Jesper Roested
CEO
Phone: +45 2122 7772
jesper@roested.dk
TJCC-Subcuject ApS
Olesvej 14
DK-2830 Virum
Denmark
VAT no: DK-4488809
Management

Jesper Roested
CEO
20+ years experience in business development, management and venture financing within medtech and life sciences. MSc. In Medical Electronics & Physics. Previously Partner at VF Venture investing in- and development of medtech companies. CEO of DDD-Diagnostic (nuclear medicine scanners), multiple positions at Novo Nordisk (in drugs and devices) and McKinsey & Co.

Claus Schmidt Møller
Inventor
B. Sc. Mechanical Engineering. 20+ years of track record in innovation of drug delivery devices. Inventor of Novopen 4 & 5 and co-inventor of Flexpen and Flextouch for Novo Nordisk. Several inventions of various medical devices made for or licensed to major Pharma and drug delivery companies. Mentioned as inventor or co-inventor on 60+ patent families.

Tomas Gundberg
Design and test engineer
Design and test engineer with more than 20 years of experience and with own development toolshop. Previous involvement in development of a large number of innovative medical devices including infusion sets and patch pumps.

Claus Demant
Extensive international experience in sales, business development and leadership from Consumer Tech & Healthcare Industries. Executive MBA, from CBS-SIMI. Global Business Development in Bang & Olufsen and European Regional Director position for Business Development at Novo Nordisk (Obesity). Mentor for startups at DTU Science Park and co-founder of several new companies